Solute and Solvent
Basically, a solution consists of both solute and solvent. So in simple words, we can say that solutes are those substances which are dissolved by the solvent in the solution and solvents are those substances that dissolve solute in the solution. We can also say that in a solution those substances having maximum quantity are called solvents and that substances which have minimum quantity are called solutes. For Example: In Solution of Sugar, sugar is the solute and water is the solvent.
Colligative Properties
Colligative Properties are defined as those properties of the solution that depend on the quantity or ratio of solute and solvent present in the solution. These properties do not affect the chemical nature of the solution (mixture of solute and solvent).
We can express the Colligative Properties using different concentrations of the solution that is Molality, Morality and Normality. With the help of colligative properties, we can understand the links between the solution and its solute concentration.
Read More: Mole Concept and Molar Mass Detailed Explanations.
Types of Colligative Properties
There are 4 Types of Colligative Properties
- Relative Lowering in Vapour Pressure.
- Elevation in Boiling Point.
- Depression in Freezing Point.
- Osmotic Pressure.
What is Colligative Property
The Colligative Properties are the Characteristics of the dilute solution which contains involatile solute (Involatile refer to do not being converted into vapour easily). These characteristics do not depend on the nature of the solute it depend upon the quantity present.
Colligative Properties are shown only after the dissolving of a non-volatile solute in the solvent. The characteristics of the solvent may change because of the taking place of the molecules by the solute which leads to a reduction of concentrations.
Colligative Property Examples
- Example 1: The Freezing point of the normal water is 0 Degree Celsius but after adding the salt in the glass full of water then the freezing point of the water goes down to low.
- Example 2: There is a relative lowering in the freezing point of the water mixed with alcohol because of their colligative properties. As not only a freezing point it also affects other properties like vapour pressure, osmotic pressure etc.
What is Vapour Pressure
Vapour Pressure refers to the process or tendency of any substance to convert into its gaseous or vapour form.
Read More: States of Matter (Solid, Liquid and Gaseous) Detailed Explanations.
Different types of colligative properties on a solution
The colligative properties shown by dilute solution after mixing of solutes are Lowering in Vapour Pressure, Boiling point elevations, Freezing point Depression and Osmotic Pressure.
Relative Lowering in Vapour Pressure

Relative Lowering in Vapour Pressure consists of a decrease in the vapour pressure of the solution in comparison to pure solvent because of the mixing of the solute.
Process: As the container having pure solvent presents its own molecules and when the solute is added to the solvent the molecules of the solute take the surface of the solvent’s molecules this leads to the reduction of some solvent molecules. So, due to this, the vapour pressure of the solution is going to decrease in comparison to pure solvent.
Let Initial vapour pressure (Vp) = Pi
Vapour Pressure after adding solvent = Pa
Moles Fraction of Solute = Xb
Then the mole fraction of the solute: Xb = Pi – Pa / Pi
Pi – Pa = Relative lowering in vapour pressure.
Let the n no. of solute be added to the N no. of solvent, then according to Raoult’s law the moles fraction of the solute is equal to:
Xb = n / n + N
So,
n / n + N = Pi – Pa / Pi
Important Point: Vapour Pressure is directly proportional to its temperature so on an increase in temperature its vapour pressure increases and on a decrease in temperature its vapour pressure decreases.
Elevation in Boiling Point

Elevation of boiling point refers to the increase in the boiling point of the solution in comparison to pure solvent when the solute is added.
Boiling Point: The boiling Point refers to the temperature where the atmospheric pressure and the vapour pressure become equal.
Process: When we add the solute in the pure solvent then we know that there is a decrease in vapour pressure takes place. So, we have to increase the temperature of the solution to reach its boiling point (in which vapour pressure is equal to atmospheric pressure). So the increase in the boiling point of the solution in comparison to boiling point of pure solvent comes under the elevation in boiling point.
For Example, Normal water has a boiling point = 100 Degree Celsius.
After adding sugar let it to boiling point = 102 Degrees Celsius.
This shows the rise in temperature of an extra 2 Degrees Celsius after adding sugar as a solute.
Tb (f): Boiling Point Final
Tb (i): Boiling Point Initial
Kb: Molal Elevation Constant
M: Molality of the solute
Δ Tb = Tb (f) – Tb (i)
∆Tb ∝ M
Δ Tb = Kb * M
Molality (M) = No of moles of solute / Mass of Solvent in Kg
Depression in Freezing Point

Depression at the Freezing point refers to a decrease in the freezing point of the solution in comparison to pure solvent when the non-volatile solute are added to the solvent.
Freezing Point: The freezing point refers to the temperature at which the vapour pressure of the substance in liquid form is equal to the vapour pressure of its solid form.
Process: According to Raoult’s law when we add solute in the pure solvent than the vapour pressure of the solution is going to decrease in comparison to the pure solvent. So due to the decrease in vapour pressure, we have to lower the temperature. So, this decrease in the freezing point of the solution in comparison to pure solvent comes under Depression in Freezing Point.
For Example: If normal water freezes at 0 Degrees Celsius and after adding sugar it freezes at -1 degrees Celsius. it always decreases.
Δ Tf = Tf (f) – Tf (i)
∆Tf ∝ M
Δ Tf = Kf * M
Tf (f): Freezing Point Final
Tf (i): Freezing Point Initial
Kf: Molal Freezing Constant / Molal Depression Constant
M: Molality of Solute
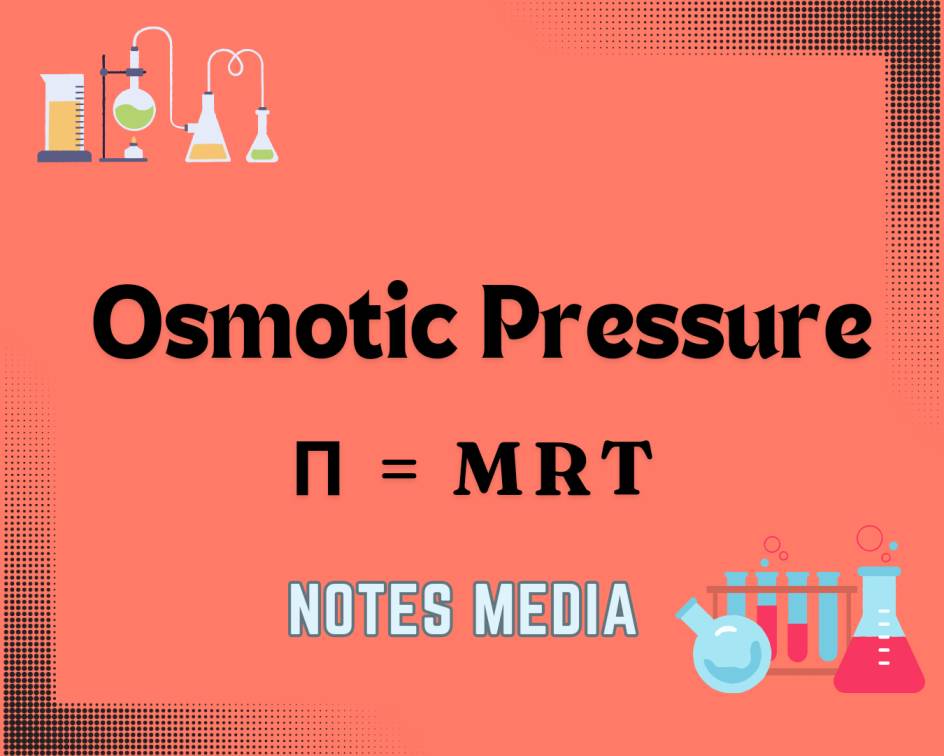
Osmotic Pressure
The net movement of the solvent molecules from solvent to solution can be stopped by applying some extra pressure over the solution side is called osmotic pressure.
For better understanding, we have to go through their process.
Semi-permeable membranes are thin membranes whose function is to pass fine particles like solvents in comparison to big particles like solutes.
Process: Take one U Shape container having solvent and solution with the semi-permeable membrane in between them. It is observed that due to the difference in vapour pressure, the solvent started going to the solution side through a semi-permeable membrane Hence, this process is called osmosis. If we apply pressure on the side of the solution to stop the coming of the solvent, then this applied pressure is called osmotic pressure.
π = MRT
π: Osmotic Pressure
M: Molarity
R: Universal Gas Constant
T: Temperature
Molarity = No. of moles of solute / Volume of Solution in Litre
n = No. of moles
No. of moles (n) = Given Mass / Molecular Mass
Social Link of Our Page
- Instagram: Click Here
- Whatsapp Community: Click Here
- YouTube: Click Here
Different Types of Solutions
Isotonic Solution: When 2 solutions having the same osmotic pressure at the same temperature these solutions are called isotonic solutions. There is no osmosis takes place between these solutions when they are separated by a semi-permeable membrane.
[ Same Osmotic Pressure (π 1 = π 2) ]
Hypotonic Solution: The Hypotonic solution is the condition when the osmotic pressure of the solution is less than its surroundings. When the Hypotonic solution is separated by the membrane than the water are comes out from the hypotonic solution.
Less than Osmotic Pressure (π 1 < π 2)
Hypertonic Solution: The Hypertonic solution is the condition when the osmotic pressure of the solution is more than the surrounding. When the Hypertonic solution is separated by the membrane than the water moves inside the solution.
More than Osmotic Pressure (π 1 > π 2)
Van’t Hoff Factor
When the solute is added to the solvent then they start going to associate and disassociate within the solution. Due to this the concentration of the solution increases or decreases which leads to changes in the colligative properties. So for measuring these changes in the property here comes the Van’t Hoff Factors.






